STERILITY Verify the sterility of every sterilized batch of medium by incubating a part of the media at the specified incubation temperature for fourteen days. No growth of microorganisms occurs.
In the end, our knowledge exhibits that it's the general efficiency of the sum of your contamination controls set up that gives a greater volume of assurance that an item is sterile.
For tissue products, a confirmation in the existence or absence of doable objectionable organisms may also be performed. Nelson Labs employs genotypic identifications using the MicroSeq system coupled with traditional Gram stain and other lab approaches.
Sterility testing makes certain the safety of products by detecting microbial contamination. For every the normal compendial methodology, products are incubated in progress media for 14 times once the Original testing is done in the cleanroom or isolator, making it possible for possible contaminants to proliferate adequately for Visible detection.
For products tested inside the isolator facility, a peroxide ingress verification need to even be performed to evaluate the decontamination system linked to putting solution into the isolator.
Particulate Evaluation testing features procedures for getting rid of, counting and sizing particulate contaminants on or in health-related units, stents, catheters, prescription drugs, IV baggage and speak to lens solutions. This exam is helpful for identifying the amount of particulate subject coming in the manufacturing natural environment and use with the healthcare system or pharmaceutical merchandise along with identifying the possible sources of particulates.
Because sterility testing is a really exacting procedure, the place asepsis in the procedure need to be ensured for an accurate interpretation of results, it is vital that staff be appropriately trained and certified. The exam for sterility is performed less than aseptic problems. So as to realize this sort of problems, the examination surroundings has to be tailored to the way in which wherein the sterility examination is carried out.
We are trying our greatest to generate This website consumer-pleasant and resourceful with timely/up to date information about Just about every pathogen, ailment due to them, pathogenesis, and laboratory diagnosis.
The necessity for sterility can differ between biopharmaceuticals. Some products, including These supposed for intravenous injection need to be proven to get sterile, although other products could have a microbial limit established. Here is the Restrict of microorganisms which the ultimate merchandise can consist of to ensure that danger to the consumer is extremely lower but that is acceptable for manufacture.
four.one.24 At the same time put together a chamber Command in the sterility take two tubes, a person is SCDM & other a single is FTM tube, unplug the cotton plug from the tube and expose in LAF for the duration of sterility, just after completion of sterility re-plug the tubes then incubate the tubes as being a chamber Handle.
[Notice—Seed-great deal society servicing procedures (seed-large amount techniques) are made use of so that the feasible microorganisms utilized for inoculation are usually not a lot more than five passages removed from the initial learn seed good deal.
This Internet site is utilizing a safety company to protect alone from on line assaults. The action you merely carried out induced the security Option. There are many steps that might trigger this block such as publishing a certain term or phrase, a SQL command or malformed information.
Plasma contains largely drinking water, electrolytes, hormones, proteins and carbon dioxide; and it provides reserve protein for the body, guards in opposition to infections and retains electrolytes well balanced. Plasma carries hormones, proteins and nutrients all through the body as wanted and gets rid of waste products; and it constitutes about fifty five website % of the full blood cells. That is why it is vital to always ensure aseptic techniques in the creation of these biologics with the Preliminary phase of manufacturing to the last stage of producing. Contamination of Organic products throughout manufacturing could final result through the staff involved with the generation system; equipments and devices employed for the output; Uncooked materials together with h2o employed for the creation; and also the creation setting or facility. The output surroundings and/or facility concerned for that manufacture of Organic products need to be as sterile as is possible (i.e. free from all feasible microorganisms capable of leading to contamination) in other to make certain the Organic products are match for animal or human usage.
Sterility testing is a significant system while in the pharmaceutical field, which makes sure that products, Primarily Those people given parenterally, are devoid more info of viable microorganisms. This method is essential to affected individual protection, product success, and compliance with regulatory specifications.
 Barret Oliver Then & Now!
Barret Oliver Then & Now! Kenan Thompson Then & Now!
Kenan Thompson Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Burke Ramsey Then & Now!
Burke Ramsey Then & Now!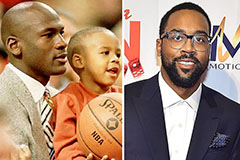 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!